Soybean is a warm-climate industrial crop, thrives in low- to medium-altitude legume crops. However, its production in Ethiopia lags behind global standards due to limited improved varieties and reliance on narrow genetic base materials, resulting in low productivity. Consequently, an experiment was undertaken to evaluate the genetic variability and associations among traits in various soybean genotypes concerning grain yield and related factors. Forty-nine soybean genotypes were assessed using a simple lattice design with two replications at Assosa Agricultural Research Center during the main cropping season of 2020. The majority of the characteristics displayed positive correlations both at phenotypic and genotypic levels. Seed yield had highly significant and positive correlations, genetically and phenotypically, with the total number of seeds/ plant, number of pods/primary branch per plant, and the weight of a hundred seeds, indicating the potential for concurrent enhancement of grain yields and these associated traits. The total number of seeds/ plant had the greatest genotypic (0.94) and phenotypic (0.51) -+direct influence on seed yield, followed by the number of pods/primary branch per plant and the weight of a hundred seeds, which showed higher genotypic direct effects on seed yield. This suggests that specific emphasis should be placed on these traits for direct selection aimed at improving yield. Moreover, through examinations of genetic diversity, it has been confirmed that there exists significant variability among the evaluated genotypes. This discovery offers valuable insights for future soybean breeding programs. The identification of such variability is crucial as it allows breeders to select and develop soybean varieties with desirable traits, ultimately contributing to the improvement and advancement of soybean varieties.
| Published in | Reports (Volume 4, Issue 3) |
| DOI | 10.11648/j.reports.20240403.14 |
| Page(s) | 54-62 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2024. Published by Science Publishing Group |
Correlation, Direct and Indirect Effect, Grain Yield
No | Genotypes Designation | Source | Year introduced | No | Genotypes Designation | Source | Year introduced |
|---|---|---|---|---|---|---|---|
1 | T44-15-T105-16-sc1 | JARC | 2016 | 26 | PB-12-8 | PARC | 2012 |
2 | PI417089A | JARC | 2016 | 27 | PM12-9 | PARC | 2012 |
3 | PI471904 | JARC | 2016 | 28 | PM12-10 | PARC | 2012 |
4 | T47-15-T126-16-SF1 | JARC | 2014 | 29 | PM12-11 | PARC | 2012 |
5 | Tgx-2008-4F | PARC | 2014 | 30 | PM12-12 | PAR | 2012 |
6 | SCS -1 (check) | JARC | 2016 | 31 | Tgx-1990-59p | PARC | 2012 |
7 | JM-CLK/CRFD-15-SD | JARC | 2016 | 32 | Gishama (Check) | PARC | |
8 | JM-ALM/H3-15-SG | JARC | 2016 | 33 | Belessa-95 (STCH) | PARC | |
9 | T34-15-T73-16-SD1 | JARC | 2016 | 34 | Tgx-1990-55F | PARC | 2014 |
10 | Tgx-2004-13F | PARC | 2014 | 35 | Tgx-2011-3F | PARC | 216 |
11 | JM-ALM/H3-15-SE1 | JARC | 2016 | 36 | Tgx-1990-57F | PARC | 2014 |
12 | JM-ALM/H3-15-SF-2 | JARC | 2016 | 37 | Tgx-1987-10F | PARC | 2014 |
13 | 5002 T | JARC | 2014 | 38 | Tgx-1935-10F | PARC | 2014 |
14 | JM-HAR/ALM-15-SB | JARC | 2016 | 39 | Tgx-2004-3F | PARC | 2016 |
15 | T34-15-T74-16-SE1 | JARC | 2014 | 40 | Tgx-1448-2F | PARC | 2016 |
16 | T34-15-T72-16-Sc1 | JARC | 2014 | 41 | Tgx-2010-3F | PARC | 2016 |
17 | JM-ALM/H3-15-SB-2 | JARC | 2016 | 42 | Tgx-1990-78F | PARC | 2013 |
18 | JM-PR142/CLK-15-SE | JARC | 2016 | 43 | Tgx-1989-19F | PARC | 2013 |
19 | PB-12-1 | PARC | 2012 | 44 | Tgx2008-2F | PARC | 2016 |
20 | PB-12-2 | PARC | 2012 | 45 | Tgx-2007-11F | PARC | 2016 |
21 | PB-12-3 | PARC | 2012 | 46 | TgX-19-87-68F | PARC | 2014 |
22 | PB-12-4 | PARC | 2012 | 47 | Tgx-2006-3F | PARC | 2016 |
23 | PB-12-5 | PARC | 2012 | 48 | Tgx-2010-12F | PARC | 2014 |
24 | PB-12-6 | PARC | 2012 | 49 | Tgx-2010-11F | PARC | 2014 |
25 | PB-12-7 | PARC | 2012 |
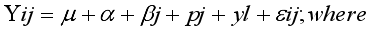
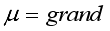 mean
mean 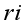 =i treatment effect,
=i treatment effect, 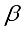 j block effect (nested with in replication),
j block effect (nested with in replication), 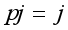 replication effect, yl =effect of 1 level of intra block error and
replication effect, yl =effect of 1 level of intra block error and 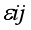 =error term.
=error term. Variable | DF | DM | PH | NBPP | NSPP | NPPP | TNSPP | NDN | FWT |
|---|---|---|---|---|---|---|---|---|---|
DF | 1.00 | 0.69** | 0.52** | -0.07ns | -0.45** | -0.07ns | -0.04ns | -0.13 | 0.17* |
DM | 0.70** | 1.00 | 0.68** | 0.02ns | -0.43** | 0.05ns | 0.07ns | -0.04ns | 0.24* |
PH | 0.56** | 0.71** | 1.00 | 0.21* | -0.34 | 0.23* | 0.17ns | 0.06ns | 0.39** |
NBPP | -0.06 | 0.07ns | 0.16 | 1.00 | 0.26* | 0.68** | 0.62** | 0.17ns | 0.37** |
NSPP | -0.51** | -0.48** | -0.40** | 0.27* | 1.00 | 0.13ns | 0.13ns | -0.01ns | -0.05 |
NPPP | -0.06ns | 0.07ns | 0.20ns | 0.6** | 0.11ns | 1.00 | 0.88** | 0.23* | 0.38** |
TNSPP | -0.03ns | 0.08ns | 0.15ns | 0.66** | 0.12ns | 0.89** | 1.00 | 0.35** | 0.24* |
NDPP | -0.15ns | -0.03ns | 0.05ns | 0.18ns | -0.02ns | 0.22ns | 0.35* | 1.00 | 0.09ns |
FWT | 0.18ns | 0.25 | 0.39* | 0.43** | -0.08ns | 0.39** | 0.23ns | 0.08ns | 1.00 |
DWT | -0.02ns | -0.02ns | -0.04ns | 0.35* | 0.17ns | 0.24ns | 0.2ns | -0.02 | 0.55** |
PLTH | -0.37** | -0.17ns | 0.06ns | 0.30* | 0.33* | 0.12ns | 0.13ns | 0.01ns | 0.24ns |
NPB | -0.06ns | 0.07ns | 0.16ns | 1** | 0.27* | 0.70** | 0.66** | 0.18ns | 0.43** |
NPPBR | -0.09ns | 0.03ns | 0.01ns | 0.82** | 0.20ns | 0.87** | 0.79** | 0.17ns | 0.41** |
NSPPBP | -0.11ns | -0.01ns | -0.04ns | 0.80** | 0.25* | 0.86** | 0.87** | 0.25* | 0.33* |
OILC | -0.41** | -0.22ns | -0.09ns | 0.21ns | 0.37* | 0.14ns | 0.08ns | -0.08ns | 0.13ns |
HSWT | -0.20ns | 0.01ns | 0.14ns | 0.03ns | 0.14ns | 0.17ns | -0.09ns | -0.22ns | 0.36* |
SYtph | -0.06ns | 0.11ns | 0.34* | 0.47** | 0.12ns | 0.64** | 0.57** | 0.15ns | 0.44** |
Variable | DWT | PLTH | NPB | NPPBR | NSPPBP | OILC | HSWT | SYtph |
|---|---|---|---|---|---|---|---|---|
DF | -0.02ns | -0.34** | -0.07ns | -0.09ns | -0.11ns | -0.36** | -0.15ns | -0.06ns |
DM | -0.02ns | -0.17 | 0.02ns | 0.01ns | -0.01ns | -0.21 | 0.04ns | 0.11ns |
PH | -0.02ns | 0.09ns | 0.21ns | 0.05ns | -0.01ns | -0.06ns | 0.15ns | 0.34** |
NBPP | 0.31** | 0.29** | 1** | 0.77** | 0.72** | 0.27* | 0.07ns | 0.41** |
NSPP | 0.15ns | 0.26* | 0.2* | 0.20* | 0.23* | 0.34** | 0.07ns | 0.11ns |
NPPP | 0.24* | 0.16ns | 0.68** | 0.87** | 0.85** | 0.17ns | 0.18ns | 0.62** |
TNSPP | 0.20* | 0.14ns | 0.62** | 0.79** | 0.86** | 0.10ns | 0.01ns | 0.57** |
NDPP | -0.01ns | 0.03ns | 0.17ns | 0.18ns | 0.25 | (-0.0ns | -0.19* | 0.15ns |
FWT | 0.55** | 0.22* | 0.37** | 0.40** | 0.33** | 0.12ns | 0.32** | 0.44** |
DWT | 1.00 | 0.27* | 0.31** | 0.35** | 0.29** | 0.23* | 0.41** | 0.25* |
PLTH | 0.29* | 1.00 | 0.29** | 0.18* | 0.18* | 0.30** | 0.35** | 0.42** |
NPB | 0.35* | 0.30* | 1.00 | 0.77** | 0.72** | 0.2* | 0.07ns | 0.41** |
NPPBR | 0.35* | 0.17ns | 0.82** | 1.00 | 0.94** | 0.20* | 0.15ns | 0.52** |
NSPPBP | 0.30* | 0.17ns | 0.80** | 0.96** | 1,00 | 0.19* | 0.07ns | 0.51** |
OILC | 0.27ns | 0.44** | 0.21ns | 0.18ns | 0.19ns | 1,00 | 0.21* | 0.25* |
HSWT | 0.46** | 0.44** | -0.03ns | 0.14ns | 0.06ns | 0.24* | 1,,00 | 0.45** |
SYtph | 0.24* | 0.49** | 0.47** | 0.53** | 0.52** | 0.27* | 0.50** | 1,00 |
Traits | PH | NPPP | TNSPP | FW | DWT | PL | NPB | NPPBR | NSPPBP | OILC | HSW |
|---|---|---|---|---|---|---|---|---|---|---|---|
PH | 0.07 | -0.01 | 0.03 | 0.11 | 0.03 | -0.01 | 0.00 | -0.11 | 0.17 | -0.01 | 0.00 |
NPPP | 0.00 | -0.16 | 0.82 | 0.11 | -0.08 | -0.01 | -0.08 | 0.73 | -0.75 | 0.02 | 0.00 |
TNSPP | 0.00 | -0.14 | 0.94 | 0.06 | -0.06 | -0.01 | -0.07 | 0.64 | -0.75 | 0.01 | -0.06 |
FW | 0.02 | -0.06 | 0.19 | 0.32 | -0.19 | 0.04 | -0.04 | 0.34 | -0.29 | 0.01 | 0.09 |
DWT | 0.00 | -0.04 | 0.16 | 0.17 | -0.36 | 0.07 | -0.04 | 0.3 | -0.24 | 0.03 | 0.17 |
PL | 0.00 | 0.01 | -0.04 | 0.04 | -0.09 | 0.26 | -0.03 | 0.04 | -0.05 | 0.04 | 0.11 |
NPB | 0.00 | -0.11 | 0.57 | 0.11 | -0.11 | 0.06 | -0.12 | 0.68 | -0.7 | 0.02 | -0.07 |
NPPBR | -0.01 | -0.14 | 0.71 | 0.13 | -0.13 | 0.01 | -0.09 | 0.85 | -0.85 | 0.02 | 0 |
NSPPBP | -0.01 | -0.14 | 0.79 | 0.1 | -0.1 | 0.02 | -0.09 | 0.81 | -0.89 | 0.02 | -0.04 |
OILC | -0.01 | -0.03 | 0.1 | 0.04 | -0.09 | 0.1 | -0.02 | 0.19 | -0.18 | 0.1 | 0.08 |
HSW | 0.00 | 0.00 | -0.16 | 0.08 | -0.17 | 0.08 | 0.02 | -0.01 | 0.09 | 0.02 | 0.37 |
Residual | 0.26 |
Traits | PH | NPPP | TNSPP | FW | DWT | PL | NPB | NPPBR | NSPPBP | OILC | HSW |
|---|---|---|---|---|---|---|---|---|---|---|---|
PH | 0.10 | 0.02 | 0.09 | 0.08 | 0.00 | 0.02 | -0.03 | 0.01 | 0.00 | -0.01 | 0.05 |
NPPP | 0.02 | 0.09 | 0.45 | 0.08 | -0.04 | 0.03 | -0.09 | 0.11 | -0.11 | 0.02 | 0.06 |
TNSPP | 0.02 | 0.08 | 0.51 | 0.05 | -0.03 | 0.03 | -0.08 | 0.10 | -0.11 | 0.01 | 0.00 |
FW | 0.04 | 0.03 | 0.12 | 0.21 | -0.09 | 0.05 | -0.05 | 0.05 | -0.04 | 0.01 | 0.11 |
DWT | 0.00 | 0.02 | 0.10 | 0.12 | -0.17 | 0.06 | -0.04 | 0.04 | -0.04 | 0.02 | 0.13 |
PL | 0.01 | 0.01 | 0.07 | 0.05 | -0.05 | 0.22 | -0.04 | 0.02 | -0.02 | 0.03 | 0.11 |
NPB | 0.02 | 0.06 | 0.31 | 0.08 | -0.05 | 0.06 | -0.13 | 0.09 | -0.09 | 0.03 | 0.02 |
NPPBR | 0.01 | 0.08 | 0.40 | 0.09 | -0.06 | 0.04 | -0.10 | 0.12 | -0.12 | 0.02 | 0.05 |
NSPPBP | 0.00 | 0.08 | 0.44 | 0.07 | -0.05 | 0.04 | -0.09 | 0.12 | -0.13 | 0.02 | 0.03 |
OILC | -0.01 | 0.02 | 0.05 | 0.03 | -0.04 | 0.06 | -0.03 | 0.02 | -0.02 | 0.10 | 0.07 |
HSW | 0.02 | 0.02 | 0.01 | 0.07 | -0.07 | 0.08 | -0.01 | 0.02 | -0.01 | 0.02 | 0.33 |
| [1] | Afework Hagos and Adam Bekele. (2018). Cost and returns of soybean production inAssosa Zone of Benishangul Gumuz Region of Ethiopia. Journal of Development and Agricultural Economics. Vol. 10(11), pp 377-383. |
| [2] | Iqbal, Z., Arshad, M., Ashraf, M., Mahmood, T. and Waheed, A., (2008). Evaluation of soybean [Glycinemax (L.) Merrill] germplasm for some important morphological traits using multivariate analysis. Pakistan Journal of Botany, 40(6), pp. 2323-2328. |
| [3] | Sharma S., Kaur M., Goyal R. and Gill B. S. (2013). Physical characteristics and nutritional composition of some new soybean (Glycine max L. Merrill) genotypes. Journal of Food Science and Technology. 51: 551-557. |
| [4] | Hea F. J and Chen J. Q. (2013). Consumption of soybean, soy foods, soy is flavones and breast cancer incidence. Differences between Chinese women and women in Western countries and possible mechanisms, Food Science and Human Wellness. 2: 35-38. |
| [5] | Central Statistical Agency of Federal Democratic Republic of Ethiopia 2000-2012. Annual report. |
| [6] | Central Statistical Authority (CSA). 2018. Agricultural sample survey, statistical volume 1 Bulletin no 586. Addis Ababa, Ethiopia. P14. |
| [7] | Mesfin, H. H., 2018. Path analysis, genetic variability and correlation studies for soybean (Glycine max (L.) Merill) for grain yield and secondary traits at Asosa, Western Ethiopia. Greener Journal of Plant Breeding and Crop Science, 6(3), pp. 35-46. |
| [8] | Balla & Ibrahim, 2017. Genotypic correlation and path coefficient analysis of soybean [Glycine max (L.) Merr.] for yield and its components. Agric Res Tech, 7(3), pp. 1-5. |
| [9] | Malik M. A., Raffi M. Y., and Mondol M. A. 2014. Morphological characterization and assessment of genetic variability, character association, and divergence in soybean mutants. The Scientific World Journal. 1-12. |
| [10] | Gomez K. A. & Gomez A. A. (1984). Statistical procedures for agricultural research, 2nd edition. New York: Wiley. 680 pp. |
| [11] | Weber, C. R. and Moorthy, B. R. 1952. Heritable and non-heritable relationship and variability of oil content and agronomic characters in the F generations of soybean crosses. Agronomy Journal, 44: 202-209. |
| [12] | Wright, S., (1921). Systems of mating. I. The biometric relations between parent and offspring. Genetics, 6(2), p. 111. |
| [13] | Dewey, D. R. and Lu, K., 1959. A correlation and path-coefficient analysis of components of crested wheatgrass seed production 1. Agronomy journal, 51(9), pp. 515-518. |
| [14] | Berhanu, H., Tesso, B. and Lule, D., 2021. Correlation and path coefficient analysis for seed yield and yield related traits in soybean (Glycine max (L.)) genotypes. Plant, 9(4), pp. 106-110. |
| [15] | Leite, W. D. S., Unêda-Trevisoli, S. H., Silva, F. M. D., Silva, A. J. D. and Mauro, A. O. D., 2018. Identification of superior genotypes and soybean traits by multivariate analysis and selection index. Revista Ciência Agronômica, 49, pp. 491-500. |
| [16] | Adetokunbo, A. D., Esla, A. S., Esther, I. N. E. G. B. E. D. I. O. N., Glory, O. E. and Ebun, A. S., 2019. Correlation between oil Content and Yield of Some early Maturing Soybean (GLYCINE MAX (L.) MERILL) Genotypes in Keffi, Nasarawa State. International Journal of Environment, Agriculture and Biotechnology, 4(3). |
| [17] | Aditya JP, Bhartiya P and Bhartiya A. (2011). Genetic variability, heritability and character association for yield and component characters in soybean [Glycine max L. Merrill]. Journal of Central European Agriculture 12(1): 27-34. |
| [18] | Akramet, S., Hussain, B. N., Al Bari, M. A., Burritt, D. J. and Hossain, M. A., (2016). Genetic variability and association analysis of soybean (Glycine max (l.) merrill) for yield and yield attributing traits. Plant Gene and Trait, 7. |
| [19] | Faisal, P. A., Hareesh, E. S., Priji, P., Unni, K. N., Sajith, S., Sreedevi, S., Josh, M. S. and Benjamin, S., (2014). Optimization of parameters for the production of lipase from Pseudomonas sp. BUP6 by solid state fermentation. Advances in Enzyme Research, 2(04), p. 125. |
| [20] | Bekele, A. and Getnet, A., (2011). Desirable traits influencing grain yield in soybean (Glycine max (L.) Merrill). Innovative Systems Design and Engineering, 2(3), pp. 14-23. |
| [21] | Faisal, M., Siddique, I. and Anis, M., (2006). An efficient plant regeneration system for Mucuna pruriens L. (DC.) using cotyledonary node explants. In Vitro Cellular & Developmental Biology-Plant, 42(1), pp. 59-64. |
| [22] | Chavan, B. H., Dahat, D. V., Rajput, H. J., Deshmukh, M. P. and Diwane, S. L., (2016). Correlation and path analysis in soybean. STUDIES, 2(9). |
| [23] | Sileshi, Y., 2019. Estimation of variability, correlation and path analysis in soybean (Glycine max (L.) Merr.) Genotypes at Jimma, South Western Ethiopia. Journal of Natural Sciences Research, 9(7), pp. 22-29. |
| [24] | Oz, M., A. Karasu, A. T. Goksoy and Z. M. Turan (2009). Interrelationships of agronomical characteristics in soybean (Glycine max) grown in different environments. Inter. J. Agric. & Biol. 11: 85-88. |
| [25] | Amogne, A., Atnaf, M. and Bantayehu, M., 2020. Correlation and path coefficient analysis in soybean [Glycine max (L.) Merrill] genotypes in dibate, North Western Ethiopia. International Journal of Scientific Engineering and Science, 4(6), pp. 1-5. |
APA Style
Fufa, W. G., Weyessa, B. (2024). Path Analysis and Correlations Among Yield and Related Traits in Different Genotypes of Soybean (Glycine Max (L.) Merill) in the Benishangul Gumuz Region, Western Ethiopia. Reports, 4(3), 54-62. https://doi.org/10.11648/j.reports.20240403.14
ACS Style
Fufa, W. G.; Weyessa, B. Path Analysis and Correlations Among Yield and Related Traits in Different Genotypes of Soybean (Glycine Max (L.) Merill) in the Benishangul Gumuz Region, Western Ethiopia. Reports. 2024, 4(3), 54-62. doi: 10.11648/j.reports.20240403.14
AMA Style
Fufa WG, Weyessa B. Path Analysis and Correlations Among Yield and Related Traits in Different Genotypes of Soybean (Glycine Max (L.) Merill) in the Benishangul Gumuz Region, Western Ethiopia. Reports. 2024;4(3):54-62. doi: 10.11648/j.reports.20240403.14
@article{10.11648/j.reports.20240403.14,
author = {Wakjira Getachew Fufa and Bulcha Weyessa},
title = {Path Analysis and Correlations Among Yield and Related Traits in Different Genotypes of Soybean (Glycine Max (L.) Merill) in the Benishangul Gumuz Region, Western Ethiopia
},
journal = {Reports},
volume = {4},
number = {3},
pages = {54-62},
doi = {10.11648/j.reports.20240403.14},
url = {https://doi.org/10.11648/j.reports.20240403.14},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.reports.20240403.14},
abstract = {Soybean is a warm-climate industrial crop, thrives in low- to medium-altitude legume crops. However, its production in Ethiopia lags behind global standards due to limited improved varieties and reliance on narrow genetic base materials, resulting in low productivity. Consequently, an experiment was undertaken to evaluate the genetic variability and associations among traits in various soybean genotypes concerning grain yield and related factors. Forty-nine soybean genotypes were assessed using a simple lattice design with two replications at Assosa Agricultural Research Center during the main cropping season of 2020. The majority of the characteristics displayed positive correlations both at phenotypic and genotypic levels. Seed yield had highly significant and positive correlations, genetically and phenotypically, with the total number of seeds/ plant, number of pods/primary branch per plant, and the weight of a hundred seeds, indicating the potential for concurrent enhancement of grain yields and these associated traits. The total number of seeds/ plant had the greatest genotypic (0.94) and phenotypic (0.51) -+direct influence on seed yield, followed by the number of pods/primary branch per plant and the weight of a hundred seeds, which showed higher genotypic direct effects on seed yield. This suggests that specific emphasis should be placed on these traits for direct selection aimed at improving yield. Moreover, through examinations of genetic diversity, it has been confirmed that there exists significant variability among the evaluated genotypes. This discovery offers valuable insights for future soybean breeding programs. The identification of such variability is crucial as it allows breeders to select and develop soybean varieties with desirable traits, ultimately contributing to the improvement and advancement of soybean varieties.
},
year = {2024}
}
TY - JOUR T1 - Path Analysis and Correlations Among Yield and Related Traits in Different Genotypes of Soybean (Glycine Max (L.) Merill) in the Benishangul Gumuz Region, Western Ethiopia AU - Wakjira Getachew Fufa AU - Bulcha Weyessa Y1 - 2024/08/20 PY - 2024 N1 - https://doi.org/10.11648/j.reports.20240403.14 DO - 10.11648/j.reports.20240403.14 T2 - Reports JF - Reports JO - Reports SP - 54 EP - 62 PB - Science Publishing Group SN - 2994-7146 UR - https://doi.org/10.11648/j.reports.20240403.14 AB - Soybean is a warm-climate industrial crop, thrives in low- to medium-altitude legume crops. However, its production in Ethiopia lags behind global standards due to limited improved varieties and reliance on narrow genetic base materials, resulting in low productivity. Consequently, an experiment was undertaken to evaluate the genetic variability and associations among traits in various soybean genotypes concerning grain yield and related factors. Forty-nine soybean genotypes were assessed using a simple lattice design with two replications at Assosa Agricultural Research Center during the main cropping season of 2020. The majority of the characteristics displayed positive correlations both at phenotypic and genotypic levels. Seed yield had highly significant and positive correlations, genetically and phenotypically, with the total number of seeds/ plant, number of pods/primary branch per plant, and the weight of a hundred seeds, indicating the potential for concurrent enhancement of grain yields and these associated traits. The total number of seeds/ plant had the greatest genotypic (0.94) and phenotypic (0.51) -+direct influence on seed yield, followed by the number of pods/primary branch per plant and the weight of a hundred seeds, which showed higher genotypic direct effects on seed yield. This suggests that specific emphasis should be placed on these traits for direct selection aimed at improving yield. Moreover, through examinations of genetic diversity, it has been confirmed that there exists significant variability among the evaluated genotypes. This discovery offers valuable insights for future soybean breeding programs. The identification of such variability is crucial as it allows breeders to select and develop soybean varieties with desirable traits, ultimately contributing to the improvement and advancement of soybean varieties. VL - 4 IS - 3 ER -